Research
Published 9 January 2024Inspiration from Nature: Molecular ‘flowers’ that bloom

Nature’s got some great tricks
Take, for instance, nature’s ability to build large molecules out of smaller pre-made molecular pieces. From four-unit haemoglobin proteins to helical DNA to semi-permeable cell membranes, nature makes ‘supramolecular assembly’ look easy. Chemists, through slow and deliberate manipulation, are trying to keep up. This Marsden-funded project led by Professors James Crowley (University of Otago), Christian Hartinger and James Wright (both University of Auckland) aimed to build a supramolecular structure, inspired by nature, that could be put to use in fields as diverse as medicine to sustainability to materials science.
Their inspiration came from flowers with petals that open and close in response to an external stimulus, such as a fading sunset. While a beautiful image, in practice, ‘supramolecular flowers’ are not so easy to germinate. Through trial and error, including various metals and ‘petal’ construction, structure and count, they eventually, successfully, designed molecular ‘flowers’ that formed large, well-defined, cage structures. The ‘petals’ of each cage were constructed from long organic molecules and are connected by two metal ions to form the flower and, crucially, can reversibly open and close in response to a stimulus (Figure 2).
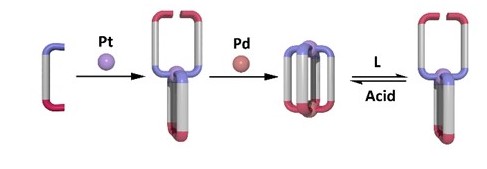
Figure 2: A cartoon representation of one of the supramolecular flowers. Addition of a platinum (Pt) precursor to a ditopic ligand, followed by a palladium (Pd) ion results in the formation of a cage structure that can be reversibly opened and closed with external stimuli
The large empty space within the molecular flower is ripe for applications like drug delivery. In this context, a molecular flower could transport a particular drug, like cancer-treating medicines, direct to its cellular target, improving treatment outcomes. While the ‘stimulus’ used in this research was pH – with ‘blossoming’ on exposure to bases and closure on exposure to acids – other stimuli could work equally as well, such as light or another chemical trigger.
This fundamental research opens up a new world of drug delivery and that of molecular transport and capture more broadly. Imagine a supramolecular flower that could selectively capture pollutants! The results have been published in high-impact scientific journals, stimulating international research discussion and allowing the work to take new directions. Professors Crowley, Hartinger and Wright are grateful to the enthusiastic students who worked on making the concept a reality. Given this important proof-of-principle study has been a success, future research will focus on building more sophisticated structures and fine-tuning their properties with the ultimate goal of realizing practical applications. The field of supramolecular chemistry will continue to blossom.
RESEARCHER
Professor Christian Hartinger
FUNDING SUPPORT
Marsden Fund
CONTRACT OR PROJECT ID
UOA1726
